It is not unusual to have some unexpected (unscheduled) bleeding in the first 6 months after starting or the first 3 months after changing MHT, even if the last natural period was many years ago. Every woman should be reviewed after their first 3 months on MHT and if the bleeding shows no signs of improving, the MHT can be adjusted to try to settle it.
Any bleeding that persists after 6 months on MHT, or starts once the patient has been on MHT for 6 months, does need to be investigated. Very heavy or painful bleeding, post-coital bleeding (after sex) or bleeding associated with other worrying symptoms would need to be investigated sooner.
A detailed history is needed to check:
- are they taking the MHT correctly e.g. do the patches stick on properly or is the patient taking the right amount of a progestogen to protect the lining of the womb?
- how long have they been on MHT and have there been any changes in treatment?
- are they using unregulated bioidentical MHT eg transdermal progesterone cream?
- when was their last smear?
- do they need a vulval, vaginal and cervical examination with swabs?
- any risk factors for endometrial cancer (see below)?
- do they need a gynaecological referral for further assessment?
We now have joint national guidelines on how to manage unscheduled bleeding on MHT (published April 2024) and local guidelines are being adapted. https://thebms.org.uk/2024/04/joint-bms-bsge-bgcs-fsrh-girft-and-rcog-guideline-on-management-of-unscheduled-bleeding-on-hrt/
There is also a summary of these guidelines available:
Most unscheduled bleeding on MHT is due to the MHT but it is very important to exclude vulval, vaginal or cervical pathology, endometrial polyps, endometrial hyperplasia, endometrial cancer, fibroids and ovarian cancer.
Risk factors for endometrial cancer include the use of unopposed estrogen in women with a uterus, obesity, Type 2 diabetes, not having children, a late menopause, polycystic ovary syndrome, previous hyperplasia and tamoxifen use. This is the 4th most common cancer in women in the UK. Women on continuous combined MHT have a lower risk of endometrial cancer than women not on MHT.
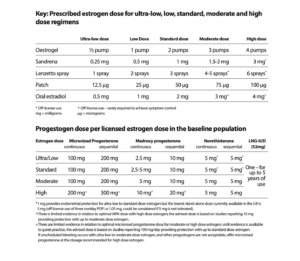
It is very important that women who still have their uterus take the progestogen part of MHT (for endometrial protection) as prescribed for as long as they are taking estrogen. Some women are reducing or stopping this part of their MHT, putting themselves at risk of endometrial cancer. The management of unscheduled bleeding on MHT guideline (link above) also includes advice on the dose of micronised progesterone or other progestogen needed depending on the dose of estrogen prescribed and any risk factors for endometrial cancer.
The new guidance on progestogen dosage below applies to all women, whether getting unscheduled bleeding or not.